From seed to patient
Max Zeller Söhne AG is one of the few companies that monitor the entire value creation process from the cultivation of the plants all the way through to the finished product.
Every year we invest around 15 per cent of our profits in research, development and quality control measures. This is vital for ensuring the quality, effectiveness and safety of our products, as well as for the development of new medications.
Learn how we get the true benefits of phyto-pharmaceuticals by controlling the seed to patient chain in the video above.


The selection process
Where it all begins
Medicinal plants form the basis of all our herbal medications.
Selection and analysis
Selecting the best plants is a time-consuming process, as it involves analysing the active ingredient profiles of numerous different varieties.
More information about how we select our medicinal plants is available on the website of our subsidiary Vitaplant AG (www.vitaplant.ch).


Cultivation of medicinal plants
Good Agricultural Practice
After the patenting process, large-scale cultivation of the plants begins in adherence with the Good Agricultural Practice guidelines.
Sustainable farming
The cultivation of our raw materials is subject to strict quality standards. We monitor every aspect that can have an impact on quality, such as the seeds and fertilisation methods, as well as the harvesting time and technique. We avoid using synthetic pesticides wherever possible.
Our seamless documentation means that our raw plant materials can be traced all the way back to the field.


Extraction
Production
During the production process, the active ingredients are extracted from the plants using a mixture of alcohol and water.
Good Manufacturing Practice
All stages of production are carried out in accordance with the pharmaceutical GMP standard (Good Manufacturing Practice) in order to ensure the greatest possible consistency and quality.

Tableting and packaging
Film-coated tablets
The dried extract is mixed with various pharmaceutical excipients and pressed into film-coated tablets.
Stability
Film-coated tablets make it possible to accurately dose the active ingredients and ensure they remain stable for long periods of time.

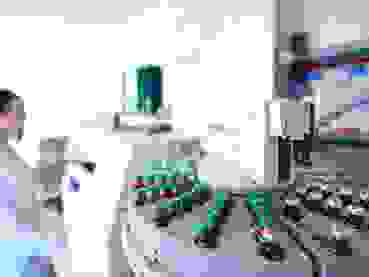
Quality control
Quality management
Quality management and quality control are among the most important competencies of any pharmaceutical company. Our products and raw materials are monitored using the latest analytical laboratory techniques.
This guarantees the safety and effectiveness of the finished products.


Research and clinical trials
Preclinical research
Before clinical trials involving test subjects can take place, the effectiveness and harmlessness of the extracts are tested in a laboratory.
Effectiveness and safety
During the clinical trials, the effectiveness and safety of the medicines is compared with that of placebos, as well as other standard treatments.
Publications
The results of these trials are regularly published in internationally recognised and independent (peer-reviewed) journals.


Approved medications
Approved medications
Public health authority
Before a medication can be sold in Switzerland, it must first be approved by Swissmedic.
Approval
Before approval is granted, the manufacturer must prove that its medication is safe, effective and economically viable.


